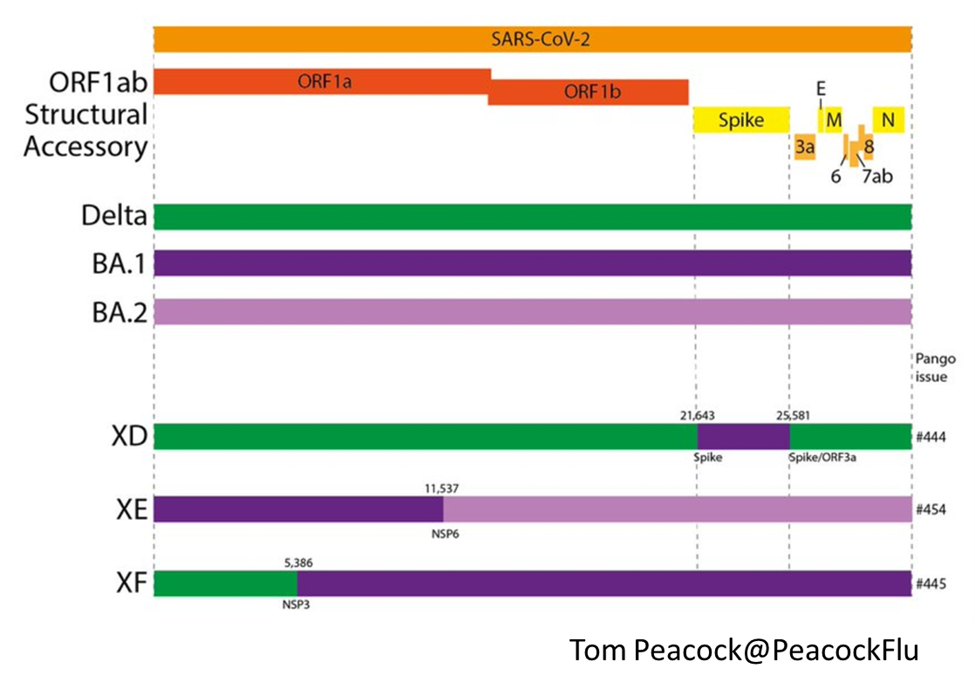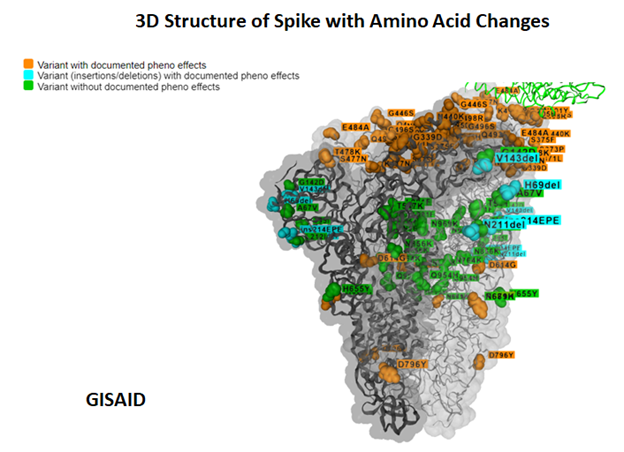
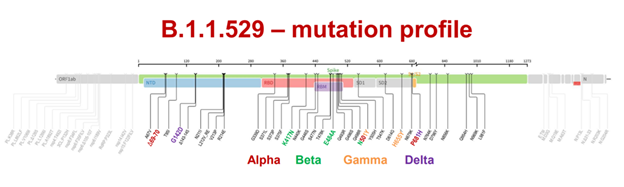
Background
SUBVARIANT BA.3
- BA.3 was originally discovered in northwestern South Africa.
- This subvariant is a combination of mutations in BA.1 and BA.2 spike proteins.
- Of the 33 mutations in the BA.3 lineage spike protein, 31 mutations are common to BA.1.
- BA.3 is currently causing the lowest number of cases in these three lineages. It may have been due to the loss of six mutations (ins214EPE, S371L, G496S, T547K, N856K, and L981F) from BA.1 or obtaining two mutations from BA.2 (S371F and D405N).
Reference
Emergence of Omicron third lineage BA.3 and its importance
Journal of Medical Virology, January 18, 2022
Efficacy of Therapeutic Antibodies
Efficacy of Antiviral Agents against the SARS-CoV-2 Omicron Subvariant BA.2
NEJM, March 9, 2022
- Both LY-CoV016 (marketed as etesevimab) and LY-CoV555 (marketed as bamlanivimab), individually and in combination, lost neutralizing activity against omicron/BA.2 (NCD1288).
- REGN10987 (marketed as imdevimab), which was previously shown to lose neutralizing activity against omicron/BA.1 (NC928) and omicron/BA.1.1 (NC929) had neutralizing activity against omicron/BA.2 (NCD1288).
- The combination of REGN10987 and REGN10933 (marketed as casirivimab) also inhibited omicron/BA.2 but did not inhibit omicron/BA.1 or omicron/BA.1.1.
- REGN10933, COV2-2196 (marketed as tixagevimab), and COV2-2130 (marketed as cilgavimab) neutralized omicron/BA.2.
- S309 (the precursor of sotrovimab) had low neutralizing activity against omicron/BA.2.
- All together, this study showed that some therapeutic monoclonal antibodies (REGN10987–REGN10933, COV2-2196–COV2-2130, and S309) have lower neutralizing activity against omicron/BA.2 than against earlier variant strains.
- The susceptibilities of omicron/BA.2 (NCD1288) to remdesivir, molnupiravir, and nirmatrelvir were similar to those of the ancestral strain and other variants of concern.
RT-PCR assays for specific detection of the Omicron variant
- Molecular detection of SARS-CoV-2 strains and differentiation of Delta variant strains
Transbound Emerging Diseases, December 29, 2021 - Specific Detection of SARS-CoV-2 B.1.1.529 (Omicron) Variant by Four RT-qPCR Differential Assays
MedRxiv, December 9, 2021 - In Silico Design of Specific Primer Sets for the Detection of B.1.1.529 SARS-CoV-2 Variant of Concern (Omicron)
Zenodo, December 1, 2021