Epsilon (B.1.427/B.1.429)
Background
- The variant known as Epsilon was first detected in California, USA
- It has two distinct lineages, B.1.427 and B.1.429, in clade 20C
- The most recent common ancestor emerged on May 20, 2020 (95% highest posterior. The branches giving rise to the B.1.427 and B.1.429 lineages were predicted to have diverged In July and June, respectively.
- Increased frequency of detection from 0% to >50% of sequenced cases from September 2020 to January.
- Each lineage rising in parallel in California as well as in multiple other states.
- An estimated increase in transmission rate of the B.1.427/B.1.429 variant relative to circulating non-B.1.427/B.1.429 lineages was 18.6-24.2%.
- A moderate resistance to neutralization by antibodies elicited by prior infection (4.0 to 6.7-fold) or vaccination (2-fold).
- One person who tested positive for CAL.20C in January of this year had previously been infected with a different SARS-CoV-2 virus, in July 2020.
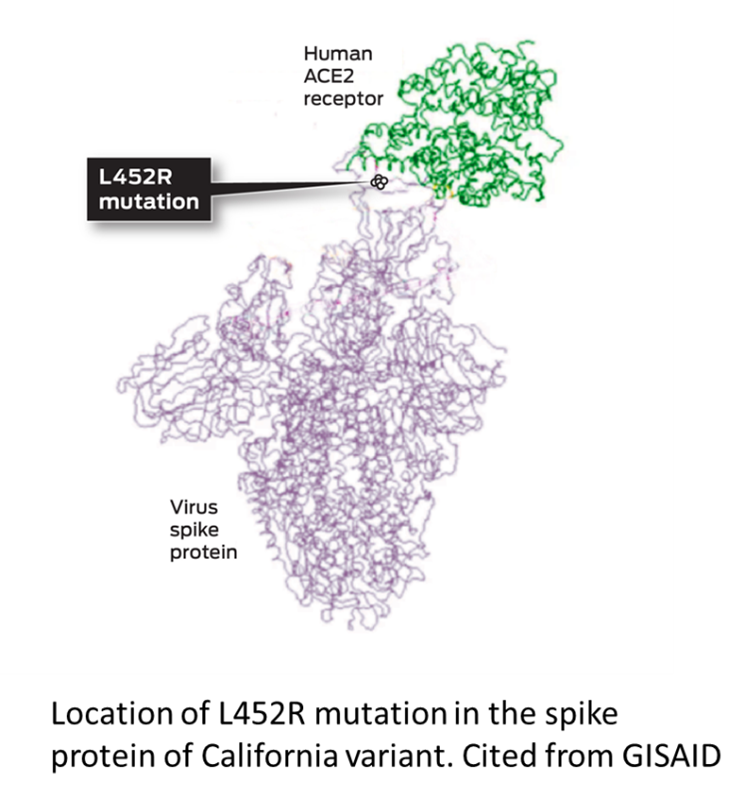
- B.1.429 variant is defined by 5 mutations: I4205V in ORF1a; D1183Y in ORF1b: S13I, W152C and L452R in the S protein.
- B.1.427 variant is defined by L452R mutation in the S protein.
- L452R mutation in the S protein locates in the receptor binding domain. L452R was first detected in minks in Denmark (March 2020).
- L452R mutation may stabilize the interaction between the spike protein and its human ACE2 receptor and thereby increase infectivity.
- Emergence of B.1.429 containing additional mutation (Q677H) in the spike protein.
- L452R mutation resulted in reduced or abolished neutralizing activity.
Disease Severity
- It is not clear whether this variant enhances the disease severity.
Therapeutic Efficacy
- Evaluation of susceptibility of variants identified through global surveillance and in subjects treated with bamlanivimab is ongoing. Pseudovirus harboring the E484K substitution had reduced susceptibility to bamlanivimab. L452R reduced bamlanivimab neutralization >1000-fold.
Vaccine Efficacy
- Analysis of neutralizing antibody responses following natural infection or mRNA vaccination has shown reduction of neutralizing antibody titers (3-6-fold) against the B.1.427/B.1.429 variant relative to wildtype pseudoviruses.
- In general, higher quality of antibody responses induced by vaccination compared to infection and their enhanced resilience to mutations against the variants.
- Vaccine efficacy against these variants needs to be confirmed by clinical trials.
Diagnostic Efficacy
- The current molecular tests detect most of the variants and thus are able to diagnose COVID-19 infection by such variants. Yet, the fine identification of the type of variants is still based on sequence analysis although multiplex PCR test are being evaluated.
- Indeed, the current variants of concern show distinctive mutations in the spike protein. Due to such mutations, most diagnostic tests for COVID-19 have been designed by targeting not only the spike protein but also other conserved proteins. For example, molecular tests designed to detect multiple SARS-CoV-2 genes (i.e., multiplex reverse transcription polymerase chain reaction targeting ORF1ab, N, and E genes) are less susceptible to the effects of genetic variation than tests designed to detect a single gene. The FDA is also monitoring the potential effects of genetic variation in molecular tests that have received Emergency Use Authorization and provides information about the tests.
- Overall, the precise characterization of the variants still relies on genomic sequencing analysis. For instance, CDC is currently increasing sequence surveillance to more than 6000 samples per week to efficiently monitor the variants of concerns and other emerging variants. COVID-19 caused by variants in the U.S. can be found here.
Publications
- Detection of the recurrent substitution Q677H in the spike protein of SARS-CoV-2 in cases descended from the lineage B.1.429
Virological, March 19, 2021 - Transmission, Infectivity, and Antibody Neutralization of an Emerging SARS-CoV-2 Variant in California Carrying a L452R Spike Protein Mutation.
BioRxiv, March 9, 2021 - Emergence of a Novel SARS-CoV-2 Variant in Southern California.
JAMA, February 11, 2021 - The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity.
Cell, July 17, 202
News
- California coronavirus variant spreading across US, threat unclear: study
Fox News, February 12, 2021 - New California Variant May Be Driving Virus Surge There, Study Suggests.
New York Times, USA, January 19, 2021
